Precision Urology: AI-Powered Kidney Tumor Detection for Early Diagnosis and Treatment
Introduction
Kidney cancer is a significant global health concern, with increasing incidence rates. Early detection is paramount for successful treatment, as localized tumors often have excellent prognosis, while advanced stages present considerable challenges. The diagnosis and characterization of kidney tumors primarily rely on medical imaging modalities such as CT and MRI scans. However, identifying and precisely delineating these lesions, especially small or complex ones, is a demanding task for radiologists, often requiring meticulous manual review of numerous image slices. This process can be time-consuming, prone to inter-observer variability, and may delay critical treatment decisions.
Artificial Intelligence, particularly through advanced computer vision and sophisticated image segmentation techniques, offers a transformative solution to these diagnostic and planning challenges. By enabling automated, precise, and consistent identification and delineation of kidney tumors from complex medical images, AI-powered kidney tumor detection promises to revolutionize clinical workflows, enhance diagnostic accuracy, and optimize patient care pathways in urology and oncology. This blog post explores our cutting-edge AI model designed for robust kidney tumor detection, highlighting its technical capabilities, profound clinical benefits, diverse applications, and the significant impact it promises for improving patient outcomes.
1. The Critical Need for Automated Kidney Tumor Detection
The urgency for efficient and accurate kidney tumor detection stems from several critical factors, directly impacting patient prognosis, treatment efficacy, and clinical resource management:
Early Diagnosis for Curability: Many kidney tumors are asymptomatic in their early stages and are often discovered incidentally during imaging for other conditions. Early and accurate detection, even of small lesions, is crucial as it significantly increases the chances of successful curative treatment and preserves renal function.
Precise Localization for Surgical Planning: For surgical interventions (e.g., partial nephrectomy, radical nephrectomy), exact localization and delineation of the tumor, including its relationship to renal vasculature and collecting system, are vital. AI provides the precision necessary for minimally invasive approaches and functional kidney preservation.
Objective Disease Characterization: Accurately measuring tumor size, volume, and tracking its growth over time provides objective data crucial for differentiating benign from malignant lesions, assessing aggressiveness, and monitoring response to non-surgical therapies.
Reducing Radiologist Workload: Manual segmentation of kidney tumors from multi-slice CT or MRI scans is extremely time-consuming for radiologists. Automation can drastically reduce this burden, allowing experts to focus on complex cases and clinical interpretation.
Enhancing Diagnostic Consistency: AI reduces inter-observer variability in tumor measurements and characterization across different radiologists and institutions, leading to more standardized and reliable diagnostic reports.
By addressing these multifaceted challenges, AI-powered kidney tumor detection is not merely a technological enhancement; it is a fundamental step towards more precise, efficient, and ultimately more effective management of kidney cancer and related renal pathologies.
2. Benefits of AI in Kidney Tumor Detection
AI-powered kidney tumor detection systems offer a multitude of transformative benefits that are reshaping radiological diagnosis and urological oncology:
Rapid and Accurate Tumor Identification: AI algorithms can analyze medical image slices instantaneously, detecting and precisely delineating kidney tumors with high accuracy. This accelerates diagnostic turnaround times, enabling quicker clinical decisions.
Precise Volumetric Analysis: By segmenting the tumor at a pixel level across all relevant slices, AI provides accurate 3D volumetric measurements. This is critical for staging, monitoring tumor growth, and assessing response to systemic therapies.
Optimized Surgical and Radiotherapy Planning: AI-generated precise tumor maps empower surgeons with enhanced pre-operative visualization for complex resections and aid radiation oncologists in delivering highly targeted radiotherapy, minimizing collateral damage to healthy kidney tissue.
Objective Monitoring of Disease Progression: AI offers consistent, quantifiable metrics of tumor size and characteristics over time, enabling objective evaluation of treatment efficacy (e.g., for targeted therapies or active surveillance protocols) and early detection of recurrence.
Reduced Clinician Fatigue and Error: Automating the laborious task of manual tumor segmentation frees up radiologists and urologists from tedious image processing, reducing the risk of errors associated with fatigue and allowing them to focus on patient-centric care.
Facilitating Research and Clinical Trials: Providing scalable and consistent quantitative imaging biomarkers derived from precise segmentation accelerates research into kidney cancer, aids in the development of new diagnostic and therapeutic strategies, and strengthens clinical trial design.
3. Data Preparation for Robust AI
The success of our kidney tumor detection model is directly attributable to the meticulous preparation of a diverse and high-quality dataset. This process involved collecting and annotating vast quantities of medical images (primarily CT and MRI scans) featuring kidneys, encompassing a wide range of patient anatomies and pathological conditions. Key aspects of our data preparation strategy included:
Multi-Modality Imaging: The dataset included scans from various imaging modalities (e.g., contrast-enhanced CT, non-contrast CT, different MRI sequences) to ensure the model’s robustness to diverse image characteristics and clinical protocols.
Diverse Tumor Types and Characteristics: Images featured various kidney tumor types (e.g., renal cell carcinoma subtypes, oncocytomas, angiomyolipomas) as well as benign lesions and cysts, ensuring the model’s ability to differentiate and detect a broad spectrum of pathologies.
Varying Tumor Sizes and Locations: The dataset encompassed tumors of different sizes (from small incidentalomas to large masses) and locations within the kidney, challenging the model to accurately delineate lesions regardless of their presentation.
Patient Anatomical Variability: Scans from diverse patient anatomies, body habitus, and presence of other abdominal pathologies were included to enhance the model’s generalization capabilities.
Expert Pixel-Level Annotation: Highly experienced radiologists and urologists meticulously delineated the boundaries of the kidney and any tumors at the pixel level on each relevant slice. This precise and detailed labeling served as the indispensable ground truth for supervised learning.
Rigorous Quality Control and Augmentation: Annotations underwent strict quality checks, and data augmentation techniques (e.g., intensity variations, noise injection, rotations, elastic deformations) were applied to artificially expand the dataset’s diversity and enhance the model’s robustness.
Model Architecture
The foundation of our advanced kidney tumor detection system is the YOLOv9m architecture. While YOLO (You Only Look Once) is primarily recognized for its efficiency in object detection, its capabilities have been extended to perform instance segmentation. This allows for pixel-level delineation of objects, making it an ideal choice for the intricate task of precisely outlining kidney tumors within complex medical image slices. The YOLOv9m variant strikes an optimal balance between high detection/segmentation accuracy and impressive inference speed, which is crucial for integration into time-sensitive clinical workflows.
Key advantages of YOLOv9m in the context of kidney tumor detection include:
Real-Time Segmentation of Medical Slices: YOLOv9m’s highly optimized design allows for extremely fast analysis of individual CT or MRI slices, enabling rapid generation of tumor masks across an entire 3D volume.
Precise Boundary Delineation: The model excels at accurately segmenting the often irregular and subtle boundaries of kidney tumors, distinguishing them from healthy kidney parenchyma, cysts, and surrounding abdominal organs at a pixel level.
Robustness to Anatomical and Pathological Variability: Its sophisticated deep learning layers are highly effective at extracting intricate features from abdominal scans, ensuring reliable performance across diverse patient anatomies and tumor characteristics.
Efficient Mask Generation for Volumetric Analysis: Beyond just identifying a tumor, YOLOv9m generates detailed pixel-level masks for each identified tumor region on each slice, providing the precise output necessary for accurate 3D volumetric reconstruction and quantitative analysis vital for clinical decision-making.
Training Parameters
The model underwent extensive training to optimize its performance across the diverse dataset. The key training parameters were carefully selected to ensure stability, rapid convergence, and robust generalization to new, unseen medical images:
Parameter |
Value |
Description |
|---|---|---|
Base Model |
YOLOv9m |
The foundational deep learning architecture employed for the task, known for its efficiency and accuracy in instance segmentation. |
Batch Size |
8 |
Number of samples processed before the model’s internal parameters are updated, balancing training stability and computational efficiency. |
Learning Rate |
0.0005 |
Controls the step size during the optimization process, a conservative rate chosen for stable convergence and fine-tuning. |
Epochs |
70 |
Number of complete passes through the entire training dataset, ensuring the model learns extensively from the data and generalizes well. |
Optimizer |
AdamW |
An adaptive learning rate optimization algorithm (Adam with decoupled weight decay) known for its efficiency and strong performance in deep learning tasks. |
Inference Time |
~0.47s |
The average time taken for the trained model to process a single medical image slice and generate a tumor segmentation mask. |
Model Evaluation
Our rigorous training and validation processes have yielded a model with robust capabilities for kidney tumor detection. The evaluation metrics below demonstrate the model’s high precision, strong recall, and overall segmentation accuracy, proving its reliability for critical applications in urology, oncology, and radiological diagnostics.
Metric |
Overall Performance |
Kidney Tumor |
Healthy Kidney Tissue/Background |
|---|---|---|---|
Precision |
0.96 |
0.95 |
0.97 |
Recall |
0.91 |
0.93 |
0.90 |
F1 Score |
0.93 |
0.94 |
0.93 |
mAP |
0.92 |
0.91 |
0.93 |
mAP@50-95 |
0.75 |
0.76 |
0.74 |
Inference Time |
~0.47s |
- |
- |
Precision (0.96 Overall): This exceptionally high precision indicates that when our model identifies pixels as belonging to a kidney tumor, it is correct 96% of the time, minimizing false positives and ensuring accurate anatomical delineation.
Recall (0.91 Overall): With a strong recall of 91%, the model successfully identifies most of the actual kidney tumor pixels in an image. This is crucial for comprehensive volumetric assessment and ensuring all parts of the tumor are captured for complete treatment planning.
F1 Score (0.93 Overall): The F1 Score, a harmonic mean of precision and recall, provides a balanced measure of the model’s overall accuracy in segmentation, reflecting its robust performance in both identifying and correctly outlining kidney tumor regions.
Mean Average Precision (mAP) (0.92 Overall): As a comprehensive metric for instance segmentation tasks across multiple classes, mAP of 0.92 signifies strong overall performance, indicating high accuracy in locating and segmenting kidney tumors and surrounding tissue.
mAP@50-95 (0.75 Overall): This metric further confirms the model’s robustness, showing good performance even at stricter Intersection over Union (IoU) thresholds, indicating precise localization of kidney tumors despite their complex and often irregular shapes.
Inference Time (~0.47s): The sub-second inference time per slice ensures that kidney tumor segmentation can be generated rapidly, enabling quick 3D reconstruction and analysis of entire patient scans for time-sensitive clinical decisions.
The per-category metrics highlight the model’s optimized performance: “Kidney Tumor” demonstrates very high recall, prioritizing the complete capture of the tumor, while “Healthy Kidney Tissue/Background” maintains excellent precision, ensuring accurate differentiation from other anatomical structures.
Epoch vs. Precision during Training
To demonstrate the training dynamics and performance stability of our model, the following graph illustrates the progression of precision over epochs. This visualization highlights how the model refined its accuracy during the training process, converging towards its optimal performance.
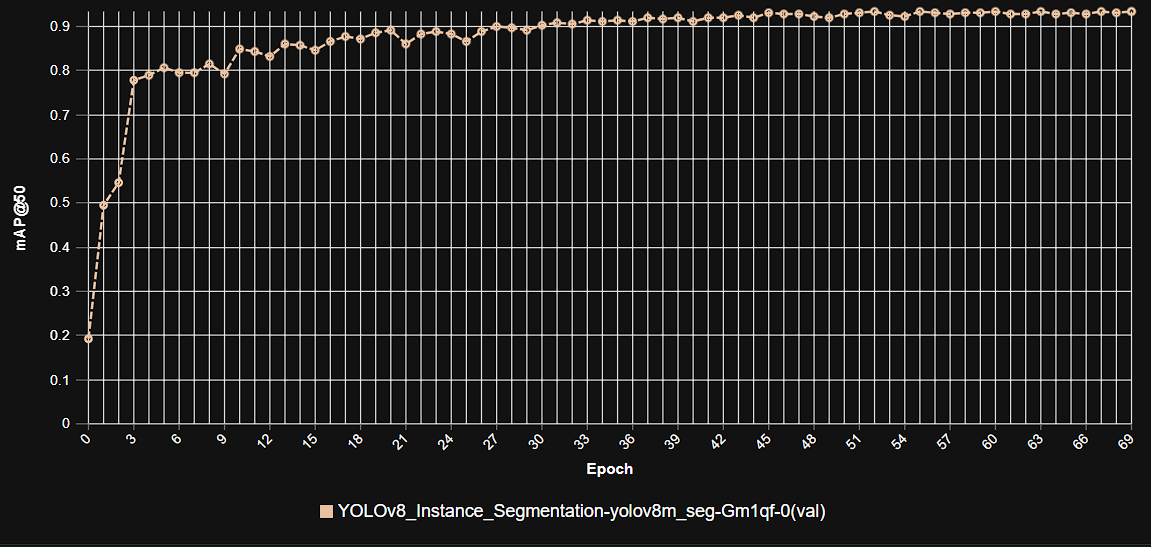 This graph illustrates the increase in model precision as training progresses over multiple epochs, showcasing the stable learning curve and high precision achieved by the YOLOv9m architecture in kidney tumor segmentation.
This graph illustrates the increase in model precision as training progresses over multiple epochs, showcasing the stable learning curve and high precision achieved by the YOLOv9m architecture in kidney tumor segmentation.
Model Inference Examples
Below are conceptual examples demonstrating the model’s output when analyzing medical images for kidney tumor detection. The AI accurately identifies and outlines the tumor within the kidney, providing precise anatomical context for clinical use.
Example 1: Precise Segmentation of a Kidney Tumor in a CT Scan
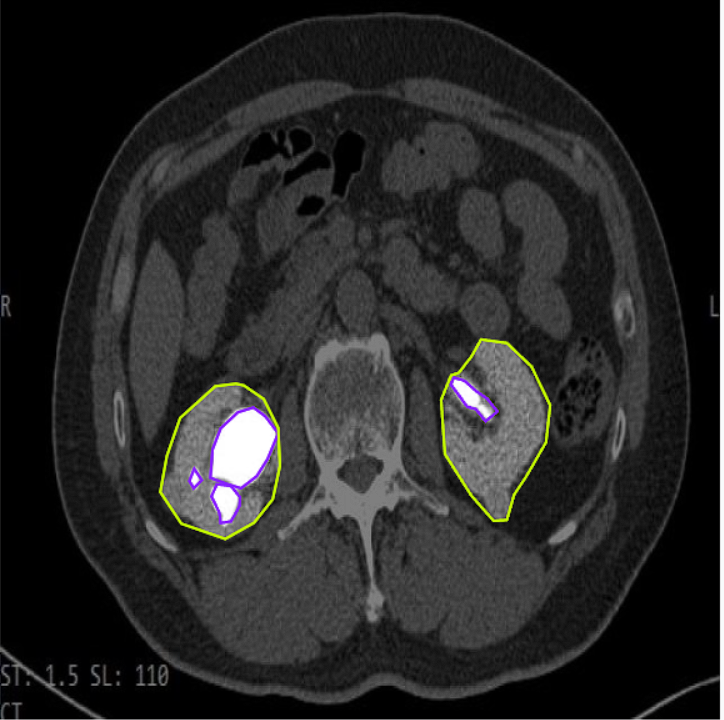 This image showcases our AI model in action, accurately delineating a kidney tumor (e.g., highlighted with a colored mask) within an abdominal CT scan slice, providing precise localization for diagnostic and planning purposes.
This image showcases our AI model in action, accurately delineating a kidney tumor (e.g., highlighted with a colored mask) within an abdominal CT scan slice, providing precise localization for diagnostic and planning purposes.
Example 2: Kidney Tumor Detection Across Different Views or Anatomies
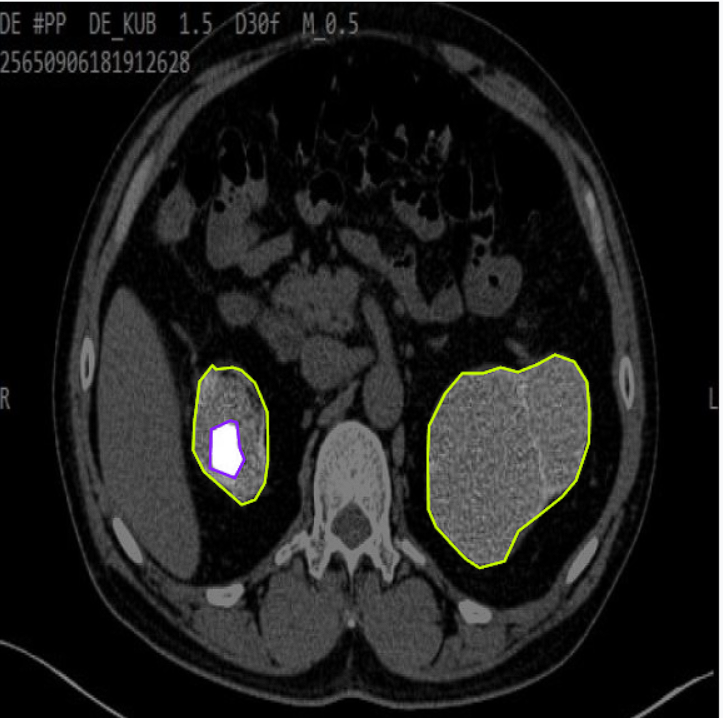 This example demonstrates the model’s versatility in detecting and segmenting kidney tumors across various patient anatomies or different slice orientations, highlighting its robust generalization capabilities.
This example demonstrates the model’s versatility in detecting and segmenting kidney tumors across various patient anatomies or different slice orientations, highlighting its robust generalization capabilities.
4. Real-World Applications and Societal Impact
The deployment of this AI-powered kidney tumor detection system is poised to create a profound impact across various medical specialties and patient care pathways in urology and oncology:
Early Diagnosis and Characterization: Aids radiologists in rapidly identifying and characterizing kidney lesions, including distinguishing between solid masses and simple cysts, leading to earlier and more accurate diagnoses.
Pre-Surgical Planning and Navigation: Provides urological surgeons with precise 3D volumetric models of tumors and their relationship to renal vasculature, enabling highly accurate planning for partial nephrectomy or radical nephrectomy.
Radiotherapy Treatment Planning: Facilitates the creation of highly accurate radiation plans for kidney cancer patients, ensuring maximum dose delivery to tumors while meticulously sparing healthy kidney tissue, thereby reducing side effects.
Quantitative Active Surveillance: For small renal masses under active surveillance, the AI provides objective, reproducible volumetric measurements, enabling precise monitoring of growth rates and informing decisions on intervention.
AI-Assisted Reporting and Workflow: Supports radiologists in rapidly reviewing and reporting on kidney scans by providing initial automated segmentations and quantitative metrics, improving efficiency and consistency in reporting.
Clinical Research and Drug Development: Provides a standardized and automated method for collecting high-throughput, quantitative imaging data for kidney cancer research, accelerating the development of new diagnostics and therapies.
5. Future Directions in AI-Powered Kidney Tumor Detection
Our commitment to innovation ensures continuous development and enhancement of our AI capabilities in kidney imaging and oncology. Future efforts will focus on:
Full 3D Volumetric Segmentation: Moving beyond slice-by-slice processing to develop truly 3D segmentation models that can process entire volumetric scans at once, improving consistency and capturing more complex tumor geometries.
Multi-Organ Segmentation: Expanding the model’s capabilities to simultaneously segment multiple abdominal organs (e.g., liver, spleen, pancreas, adrenal glands) within a single scan for comprehensive anatomical mapping and assessment of tumor spread.
Integration with Clinical Decision Support Systems: Embedding segmentation outputs directly into AI-powered tools that assist clinicians with diagnosis, prognosis prediction, and personalized treatment recommendations for kidney cancer.
Real-time Intraoperative Guidance: Developing models capable of real-time segmentation and tracking of the kidney and tumor during minimally invasive surgeries, providing augmented reality guidance for surgeons.
Prediction of Tumor Histology/Aggressiveness: Integrating image-based features from segmentation with clinical data and radiomics to predict tumor histology (e.g., clear cell vs. papillary RCC) or aggressiveness non-invasively.
Conclusion
AI-powered kidney tumor detection represents a transformative leap forward in urological oncology and medical imaging. By delivering unparalleled accuracy, efficiency, and quantitative insights into renal pathologies, our solution empowers clinicians to make more precise diagnoses, plan more effective interventions, and ultimately improve outcomes for patients facing kidney tumors. As we continue to refine and expand these capabilities, the future promises an even more intelligent, data-driven, and personalized approach to kidney cancer care.