Microscopic Precision: AI-Powered Malaria Parasite Detection for Global Health
Introduction
Malaria, a life-threatening disease caused by parasites transmitted through mosquito bites, remains a devastating global health challenge, particularly in sub-Saharan Africa. Despite significant progress, hundreds of millions of cases and hundreds of thousands of deaths are reported annually, predominantly among children. Accurate and timely diagnosis is paramount for effective treatment, preventing disease transmission, and monitoring drug resistance. The gold standard for malaria diagnosis has traditionally been microscopic examination of blood smears, a method highly dependent on the skill and experience of the microscopist, often slow, and prone to error, especially in resource-limited settings with high patient volumes.
Artificial Intelligence, driven by advanced computer vision techniques, offers a transformative solution to these diagnostic hurdles. By enabling rapid, precise, and automated identification of malaria parasites within microscopic blood images, AI is set to revolutionize malaria diagnostics, accelerate treatment initiation, and bolster global disease surveillance efforts. This blog post explores our cutting-edge AI model designed for accurate malaria parasite detection, highlighting its technical capabilities, profound clinical benefits, diverse applications, and the significant impact it promises for global public health.
1. The Critical Need for Automated Malaria Parasite Detection
The urgency for efficient and accurate malaria parasite detection stems from several critical factors, directly impacting patient outcomes and global health initiatives:
Saving Lives Through Rapid Treatment: Early and accurate diagnosis of malaria is crucial for initiating prompt antimalarial treatment, which can significantly reduce the risk of severe illness, complications, and death. Delays in diagnosis, particularly for severe malaria, can be fatal.
Controlling Disease Transmission: Identifying infected individuals quickly allows for immediate treatment, breaking the chain of transmission and preventing further spread of the disease within communities. This is vital for malaria elimination efforts.
Alleviating Workload for Diagnosticians: In endemic regions, microscopists face immense pressure due to high volumes of samples, often working long hours under demanding conditions. This can lead to fatigue, reduced accuracy, and diagnostic backlogs. AI can drastically reduce this burden, freeing up human experts for complex cases.
Addressing Shortage of Skilled Personnel: Many remote and rural areas lack access to trained microscopists, making accurate diagnosis a significant challenge. Automated systems can democratize access to high-quality diagnostics where expertise is scarce.
Monitoring Drug Resistance and Parasite Density: Automated systems can not only detect the presence of parasites but also quantify parasite density (parasitemia), which is crucial for assessing disease severity and monitoring patient response to treatment, as well as detecting trends in drug resistance.
By addressing these multifaceted challenges, AI-powered malaria parasite detection is not merely a technological convenience; it is a vital tool in the ongoing fight against one of the world’s most enduring infectious diseases, with the potential to save countless lives and accelerate global malaria elimination.
2. Benefits of AI in Malaria Diagnostics
AI-powered malaria parasite detection systems offer a multitude of transformative benefits that are reshaping clinical and public health strategies:
Accelerated Diagnosis and Treatment Initiation: AI algorithms can analyze microscopic blood smear images in seconds, providing near-instantaneous diagnostic results. This rapid turnaround time is critical for initiating timely antimalarial treatment, which directly impacts patient recovery and survival rates.
Enhanced Accuracy and Consistency: Unlike manual microscopy, which can be influenced by human fatigue, experience level, or subjective interpretation, AI models provide highly objective and consistent diagnostic outputs. Trained on vast, diverse datasets, these systems can reliably identify parasites even at low densities, reducing false negatives and positives.
Reduced Workload and Optimized Lab Resources: By automating the screening and initial detection of parasites, AI significantly reduces the manual burden on laboratory technicians and microscopists. This allows skilled personnel to focus on confirmatory diagnoses, quality control, and more complex cases, optimizing laboratory efficiency.
Bridging Accessibility Gaps in Remote Areas: AI-powered diagnostic tools can be deployed in resource-limited settings or integrated with mobile microscopy solutions. This expands access to high-quality malaria diagnosis in remote clinics and rural communities where traditional laboratory infrastructure and expert personnel are scarce.
Robust Disease Surveillance and Epidemiology: Automated detection systems can provide consistent, standardized data on malaria prevalence and parasite density across large populations. This valuable epidemiological data is crucial for public health agencies to track disease trends, identify outbreaks, and allocate resources effectively for control and elimination programs.
3. Data Preparation for Robust AI
The success of our malaria parasite detection model is directly attributable to the meticulous preparation of a diverse and high-quality dataset. This process involved collecting and annotating vast quantities of microscopic blood smear images, encompassing a wide range of real-world clinical and laboratory conditions. Key aspects of our data preparation strategy included:
Diverse Parasite Stages and Species: The dataset included images with various developmental stages of Plasmodium parasites (e.g., ring forms, trophozoites, schizonts, gametocytes) and, where applicable, different Plasmodium species to ensure comprehensive detection capabilities.
Varying Parasitemia Levels: Images ranged from very low parasite densities (challenging for human eyes) to high densities, enabling the model to accurately detect infections across the entire spectrum of parasitemia.
Different Staining Techniques: The dataset incorporated images stained using various common methods (e.g., Giemsa staining), ensuring the model’s robustness to variations in slide preparation and staining protocols.
Diverse Cell Densities and Image Quality: Images included varying red blood cell densities, backgrounds, and magnifications, along with realistic levels of image noise or artifacts, mimicking real-world microscopic slide quality.
Pixel-Level and Bounding Box Annotation: Experienced parasitologists meticulously annotated individual parasites within the red blood cells, providing precise pixel-level masks and bounding boxes. This detailed labeling served as the indispensable ground truth for supervised learning.
Model Architecture
The foundation of our advanced malaria parasite detection system is the YOLOv9m architecture. While YOLO (You Only Look Once) is primarily recognized for its efficiency in object detection, its capabilities have evolved to include instance segmentation, which is ideal for delineating and classifying tiny objects like parasites at a pixel level within microscopic fields. This specific variant was chosen for its optimal balance of high accuracy and impressive inference speed, making it perfectly suited for high-throughput diagnostic applications.
Key advantages of YOLOv9m in the context of malaria parasite detection include:
Real-Time Processing: YOLOv9m’s highly optimized design allows for extremely fast analysis of microscopic blood smear images, enabling near-instantaneous detection and classification of parasites, crucial for rapid diagnosis in clinical settings.
Detection of Tiny Objects: Malaria parasites, especially early ring stages, are very small. YOLOv9m excels at detecting and segmenting such minute objects accurately within a dense background of blood cells.
Robust Feature Extraction from Microscopic Images: The model’s sophisticated deep learning layers are highly effective at extracting intricate features from stained blood cells, allowing it to differentiate between parasites and artifacts or normal cell components with high precision.
Efficient Instance Segmentation: Beyond just identifying a parasite, YOLOv9m generates detailed pixel-level masks for each identified parasite, providing precise location and morphological information for accurate quantification (e.g., parasitemia count).
Training Parameters
The model underwent extensive training to optimize its performance across the diverse dataset. The key training parameters were carefully selected to ensure stability, rapid convergence, and robust generalization to new, unseen blood smear images:
Parameter |
Value |
Description |
|---|---|---|
Base Model |
YOLOv9m |
The foundational deep learning architecture employed for the task, known for its efficiency and accuracy in instance segmentation. |
Batch Size |
8 |
Number of samples processed before the model’s internal parameters are updated, balancing training stability and computational efficiency. |
Learning Rate |
0.0005 |
Controls the step size during the optimization process, a conservative rate chosen for stable convergence and fine-tuning. |
Epochs |
70 |
Number of complete passes through the entire training dataset, ensuring the model learns extensively from the data and generalizes well. |
Optimizer |
AdamW |
An adaptive learning rate optimization algorithm (Adam with decoupled weight decay) known for its efficiency and strong performance in deep learning tasks. |
Inference Time |
~0.45s |
The average time taken for the trained model to process a single microscopic blood smear image and output a diagnosis. |
Model Evaluation
Our rigorous training and validation processes have yielded a model with exceptional capabilities for malaria parasite detection. The evaluation metrics below demonstrate the model’s high precision, outstanding recall, and overall accuracy, proving its reliability for critical diagnostic applications in public health.
Metric |
Overall Performance |
Parasitized Cells |
Uninfected Cells |
|---|---|---|---|
Precision |
0.91 |
0.90 |
0.95 |
Recall |
0.96 |
0.97 |
0.98 |
F1 Score |
0.93 |
0.93 |
0.96 |
mAP |
0.87 |
0.85 |
0.90 |
Inference Time |
~0.45s |
- |
- |
Precision (0.91 Overall): This indicates that when our model identifies a cell as parasitized, it is correct 91% of the time, minimizing false positives that could lead to unnecessary treatment or patient anxiety.
Recall (0.96 Overall): With an exceptional recall of 96%, the model successfully identifies almost all actual parasitized cells present in an image. This metric is paramount in medical diagnostics, as missing a true positive (false negative) in malaria can have severe consequences for the patient and public health.
F1 Score (0.93 Overall): The F1 Score, a harmonic mean of precision and recall, provides a balanced measure of the model’s accuracy, reflecting its robust performance in both identifying and correctly classifying malaria parasites.
Mean Average Precision (mAP) (0.87 Overall): As a comprehensive metric for object detection and segmentation tasks, mAP of 0.87 signifies strong overall performance across all identified classes (parasitized and uninfected cells), indicating high accuracy and reliability in generating precise masks for parasite detection.
Inference Time (~0.45s): The sub-second inference time ensures that diagnoses can be generated almost instantaneously, making the system highly practical for high-volume laboratories and point-of-care settings.
The per-category metrics provide further insight: “Parasitized Cells” demonstrate a very high recall, prioritizing the detection of actual infections, while “Uninfected Cells” show excellent performance, reflecting the model’s ability to confidently identify healthy cells.
Epoch vs. Precision during Training
To demonstrate the training dynamics and performance stability of our model, the following graph illustrates the progression of precision over epochs. This visualization highlights how the model refined its accuracy during the training process, converging towards its optimal performance.
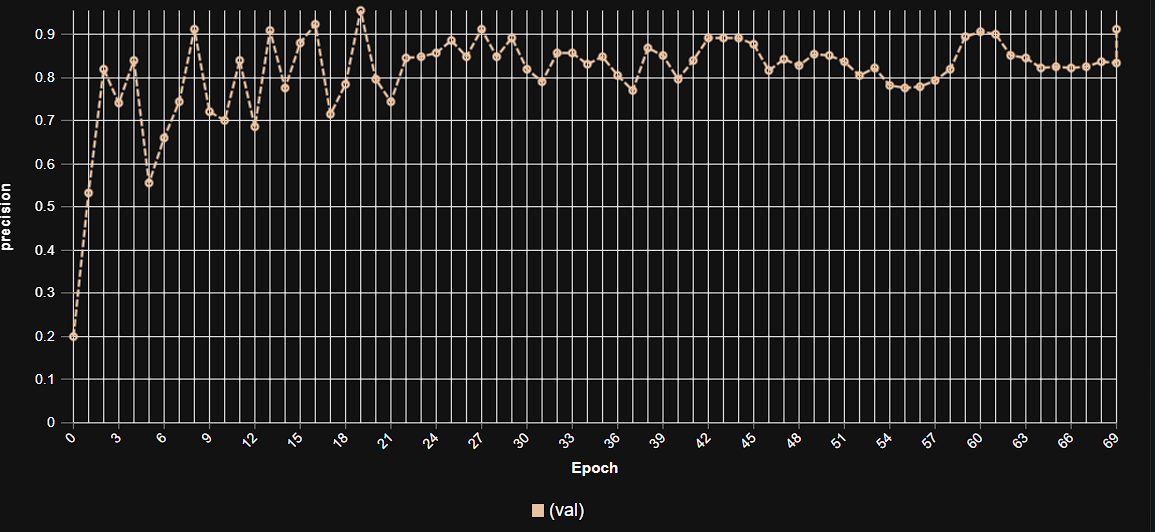 This graph illustrates the increase in model precision as training progresses over multiple epochs, showcasing the stable learning curve and high precision achieved by the YOLOv9m architecture in malaria parasite detection.
This graph illustrates the increase in model precision as training progresses over multiple epochs, showcasing the stable learning curve and high precision achieved by the YOLOv9m architecture in malaria parasite detection.
Model Inference Examples
Below are conceptual examples demonstrating the model’s output when analyzing microscopic blood smear images for malaria parasite detection. The AI precisely identifies and outlines individual parasitized cells, aiding microscopists in their diagnostic workflow.
Example 1: Detection of Ring-Stage Parasites in Red Blood Cells
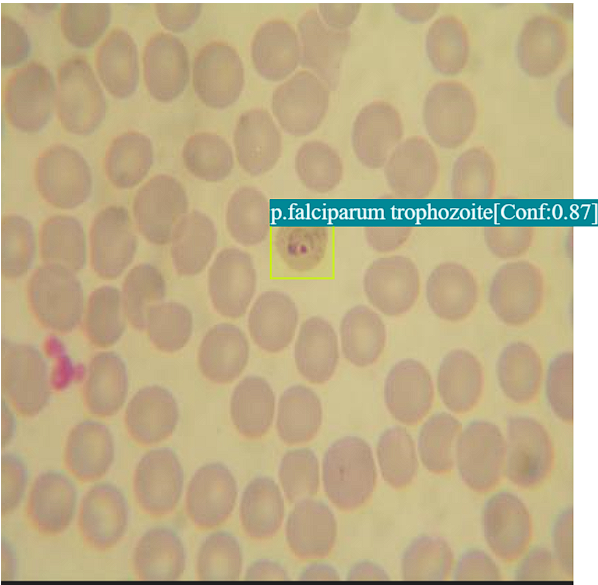 This image showcases our AI model in action, accurately identifying and highlighting ring-stage malaria parasites within red blood cells, demonstrating its capability for early detection.
This image showcases our AI model in action, accurately identifying and highlighting ring-stage malaria parasites within red blood cells, demonstrating its capability for early detection.
Example 2: Identification of Multiple Parasite Stages at Moderate Parasitemia
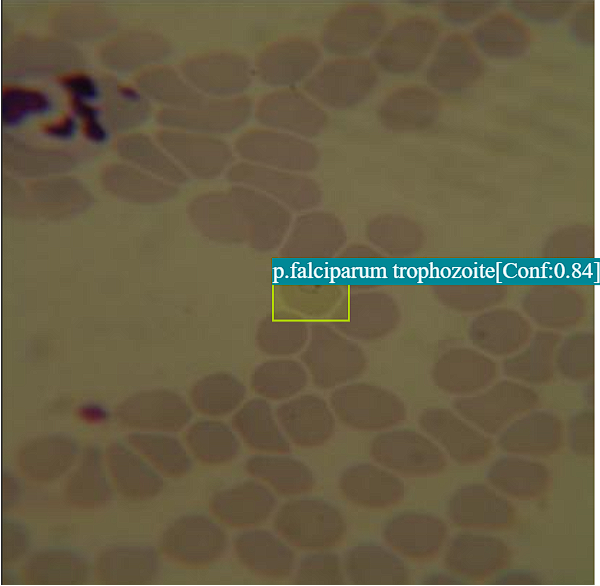 This example illustrates the model’s ability to detect various developmental stages of malaria parasites (e.g., trophozoites, schizonts) at different densities, providing comprehensive diagnostic insights.
This example illustrates the model’s ability to detect various developmental stages of malaria parasites (e.g., trophozoites, schizonts) at different densities, providing comprehensive diagnostic insights.
4. Real-World Applications and Societal Impact
The deployment of this AI-powered malaria parasite detection system is poised to create a transformative impact across various public health and clinical scenarios:
Point-of-Care Diagnostics in Remote Clinics: Integrating the AI with low-cost digital microscopes can enable rapid, accurate malaria diagnosis directly in rural and remote health centers, accelerating treatment in areas with limited access to central labs.
Mass Screening and Surveillance Campaigns: The system’s speed and scalability make it ideal for large-scale screening efforts in endemic regions, facilitating active case finding and informing targeted public health interventions.
Tele-Microscopy and Remote Diagnostic Hubs: Allows images from remote sites to be uploaded and analyzed by AI, with results reviewed by a central expert, bridging geographical gaps in diagnostic expertise.
Quality Control in Clinical Laboratories: The AI can serve as an independent verification tool for manual diagnoses, improving overall accuracy and consistency across different laboratories and ensuring high diagnostic standards.
Training and Education for Microscopists: The system can be used as an interactive educational tool, helping new microscopists learn to identify and differentiate malaria parasites by providing immediate feedback on their observations.
Drug Resistance Monitoring: By accurately quantifying parasite density and potentially identifying specific parasite characteristics, the AI can assist in monitoring the effectiveness of antimalarial drugs and detecting emerging resistance patterns.
5. Future Directions in AI-Powered Malaria Detection
Our commitment to innovation ensures continuous development and enhancement of our AI capabilities in infectious disease diagnostics. Future efforts will focus on:
Multi-Parasite and Pathogen Detection: Expanding the model to simultaneously detect and classify multiple blood-borne parasites (e.g., Trypanosoma, Leishmania) or other blood pathogens, leading to broader diagnostic platforms.
Mobile Microscopy Integration: Further optimizing models for deployment on smartphone-attachable microscopes, making highly portable and affordable diagnostic tools accessible for field workers and community health volunteers.
Quantification of Parasite Load (Parasitemia): Enhancing the model’s ability to precisely count parasites and estimate parasitemia levels, which is crucial for assessing disease severity, guiding treatment, and monitoring response.
Explainable AI (XAI) for Clinical Trust: Developing models that can not only classify but also highlight the specific visual features within the blood smear that led to the diagnosis, fostering greater trust and clinical adoption.
Integration with Clinical Data: Combining image-based AI findings with patient clinical data (e.g., fever, travel history) to build more comprehensive diagnostic and prognostic tools.
Conclusion
AI-powered malaria parasite detection represents a pivotal advancement in global health. By delivering unparalleled accuracy, speed, and accessibility, our solution empowers healthcare systems to make earlier, more precise diagnoses, fundamentally changing the landscape of malaria control. This not only translates to more effective treatment and reduced disease transmission but, most importantly, to saving countless lives and accelerating the world’s progress towards malaria elimination. As we continue to refine and expand these capabilities, the future promises a more equitable and efficient battle against infectious diseases worldwide.